Research
My work lies at the interface between physics and biology and the techniques involved range from optical engineering to visual psychophysics.
Projects
Links
Vision Restoration
The debilitating vision loss caused by photoreceptor degenerations like AMD and retinitis pigmentosa represents a significant burden on both the individuals affected and society as a whole. If identified early it may be possible to slow the rate of photoreceptor loss, but once photoreceptors have degenerated, little can currently be done to restore high quality vision that is useful for tasks such as recognising faces or reading text.
To address this challenge, I am using adaptive optics technology to optimize vision restoration therapies at the fovea, the retinal structure that is specialised to perform high acuity tasks. With its unusual anatomy and physiology, this region dominates the representation of the visual world in cortex, and prior efforts to restore vision have been most successful if this location is included. Furthermore, the fovea is the location of vision loss in the large and growing patient population with AMD.
My current work focusses on optogenetic and photoreceptor replacement therapies, but many of the specific challenges caused by the unusual anatomy and physiology of the fovea are common to all vision restoration approaches.
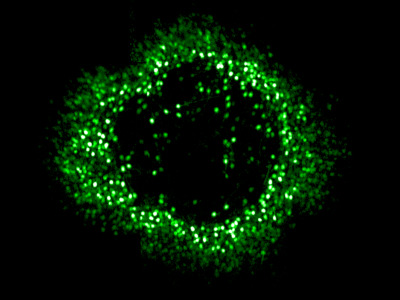
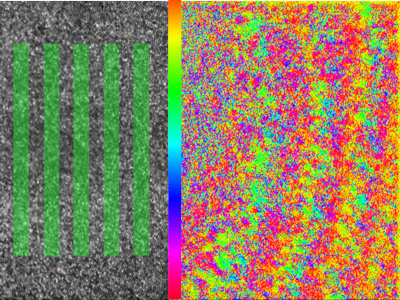
Optogenetic therapy
Even after photoreceptors have degenerated the ganglion cells, the output neurons of the retina, often persist - can we make these cells light sensitive? This is the idea behind optogenetic vision restoration, where ‘optogenes’ cause light sensitive proteins to be expressed in cells that don’t normally have them and so these cells can be used as photoreceptors instead.
Adaptive optics calcium imaging allows us to evaluate and improve these vision restoration strategies in the living eye by recording activity in foveal retinal ganglion cells. Using the light gated channel ‘ChrimsonR’ we have demonstrated restored responses in foveal ganglion cells that have lost their photoreceptor input and shown that the expected patterns of activity are generated in response to patterned visual stimuli. We are currently investigating the longevity of optogenetic vision restoration in vivo and evaluating the potential impact of retinal remodelling after photoreceptor loss.
Photoreceptor replacement therapy
To restore naturalistic vision with normal retinal processing it would be desirable to restore vision at the level of the photoreceptors themselves. This requires not only delivering photoreceptor precursor cells capable of maturing and forming synaptic connections into the sub-retinal space, but also that patient’s bipolar cell synaptic partners and downstream processing are functionally intact despite many years of photoreceptor loss.
In collaboration with David Gamm at the University of Wisconsin we have been tracking the survival and distribution of fluorescently tagged photoreceptor precursor transplants in the living eye.
Understanding the Fovea
Despite its importance, relatively little is known about the physiology of the fovea because its delicate structure makes it challenging to study electrophysiologically. At Rochester we have been using our unique adaptive optics calcium imaging platform to investigate the fovea in vivo.
By performing calcium imaging while also using the AO system to present high resolution, precisely stabilized visual stimuli, we have been able to map the location of hundreds of retinal ganglion cell (RGC) receptive fields at the fovea. The spatial arrangement of the cone photoreceptors is well preserved in the spatial arrangement of RGCs, despite the dramatic developmental changes which generate the specialized foveal structure. This orderly mapping is of relevance to vision restoration therapies that aim to directly stimulate the retinal ganglion cells themselves. We are also beginning to classify the retinal ganglion cell types found in the fovea using coloured stimuli.
I have a longstanding interest in the physiological optics of the fovea. Extended photoreceptor axons ‘Henle Fibres’ radiate from the foveal cones to their displaced bipolar cells and interact with macular pigment molecules to produce a radially symmetric polarizing filter which mediates the human perception of the polarization state of light. This visual effect is known as ‘Haidinger’s brushes’. I’ve previously explored the threshold and rotational dynamics of the phenomenon using psychophysical testing and am interested in the optical interactions which give rise to it. I often use Haidinger’s brushes in outreach activities – almost everyone can discern the polarization state of light with the naked eye!

Biological Imaging
As a PhD student at the Cavendish laboratory at Cambridge University I explored potential applications of Variable Pressure or 'Environmental' Scanning Electron Microscopy as a method for imaging dynamic biological processes. I did this work under the supervison of Prof. Dame Athene Donald FRS in the Biological and Soft Systems Sector of the Physics Department. In 2011 I was commissioned by the BBC to produce high definition images of the closure of stomatal pores using the new techniques developed during my PhD, for the BBC 2 documentary 'How to Grow a Planet'. If you are interested in learning more about VP ESEM I recommend taking a look at 'Principles and Practice of Variable Pressure: Environmental Scanning Electron Microscopy(VP-ESEM) by Dr Debbie Stokes.
Publications
Optogenetic restoration of retinal ganglion cell activity in the living primate,
McGregor J.E. et al., Nature Communications 11, 1703 (2020) DOI:10.1038/s41467-020-15317-6Imaging transplanted photoreceptors in living nonhuman primates with single cell resolution,
Aboualizadeh E.*, Phillips M.J.*, McGregor J.E.*, DiLoreto Jr, D.A., Strazzeri J. M., Dhakal K.R., Bateman B., Jager L.D., Nilles K.L, Stuedemann S.A., Ludwig A.L., Hunter J.J., Merigan W.H., Gamm D.M., and Williams D.R., Stem Cell Reports, (2020) DOI:10.1016/j.stemcr.2020.06.019 (*joint first authors)Localized Photoreceptor Ablation Using Femtosecond Pulses Focused With Adaptive Optics,
Dhakal K R., Walters S., McGregor J.E., Schwarz C., Strazzeri J., Aboualizadeh E., Bateman B., Huxlin K. R., Hunter J.J., Williams, D.R., and Merigan W. H. Translational Vision Science and Technology, 9(7):16, (2020) DOI:10.1167/tvst.9.7.16Restoring vision at the fovea,
McGregor J.E., Current Opinion in Behavioural Sciences 30, 210 - 216 (2019) DOI:10.1016/j.cobeha.2019.10.003Functional Assessment of Vision Restoration,
McGregor, J.E., Williams D.R., and Merigan W.H., Retinal Degenerative Diseases. Springer, 145 - 149 (2019) DOI:10.1007/978-3-030-27378-1_24Functional architecture of the foveola revealed in the living primate,
McGregor J.E., Yin L., Yang Q., Godat T., Huynh K.T., Zhang J., et al., PLoS ONE 13(11): e0207102. (2018) DOI:10.1371/journal.pone.0207102Perceiving polarization with the naked eye: characterization of human polarization sensitivity.
Temple S.E.*, McGregor J.E.*, Miles C., Graham L., Miller J., Buck J., Scott-Samuel N.E., and Roberts N.W., Proc. R. Soc. B, 282; DOI:10.1098/rspb.2015.0338 (2015) (*joint first authors)-
Post-processing strategies in image scanning microscopy,
McGregor J.E.*, Mitchell C.A.* and N.A. Hartell, Methods, DOI: 10.1016/j.ymeth.2015.05.002 (2015) (*joint first authors) -
Human polarization sensitivity, Chapter 14 in Polarized Light and Polarization Vision in Animal Sciences,
McGregor J.E., Temple S.E., Editor: Gábor Horváth, 2nd edition, Springer Series in Vision Research, volume 2 Editors: Shaun Collin and Justin Marshall, 303 - 315 Springer-Verlag (2014) -
ESEM Imaging in cell biology, Cell Imaging Techniques: Methods and Protocols 2nd ed.,
McGregor J.E., Staniewicz L.T.L., Guthrie S.E. and Donald A.M., Editors: D. Taajes and J. Roth. (Methods in molecular biology series), 931 493-516 Humana Press. (2012) -
ESEM imaging of dynamic biological processes: the closure of stomatal pores,
McGregor J.E. and Donald A.M., Journal of Microscopy, 239(2) 135-141 (2010) -
‘Microscopy of myelination’. in: Microscopy: Science, Technology, Applications and Education.
McGregor J.E., Wang Z., ffrench Constant C.C. and Donald A.M., Editors: Antonio Méndez-Vilas, Jesús Díaz Álvarez, 1185 - 1195 Formatex Research Center (2010)